ESPE2014 Free Communications Pituitary (6 abstracts)
Management of Hyperhydration in a Child with Syndrome of Inappropriate Antidiuretic Hormone Secretion (Siadh) Using a Selective Vasopresin Receptor Agonist
Violeta Delgado-Carballar , Ajay Thankamony , Ruben Willemsen , Daniela Elleri & David Dunger
Department of Paediatrics, University of Cambridge, Cambridge, UK
Background: Management of SIADH is challenging and no optimal therapies are available in children. We present a case of an 11 years old boy with severe SIADH resistant to 30% fluid restriction in the context of an intracranial suprasellar high grade B-cell lymphoma who requires hyperhydration for chemotherapy protocol. Tolvaptan is an oral highly selective arginine vasopressin V2 receptor antagonist, which has been approved for use in SIADH in adults.
Objective and Hypotheses: The aim was to use Tolvaptan in a patient with SIADH to treat hyponatremia and enable hyperhydration.
Method: Tolvaptan was started at a dose of 0.14 mg/kg once a day and titrated up to 0.28 mg/kg twice a day based on fluid requirements and serum sodium levels. Frequent monitoring of serum/urine electrolytes and osmolality, fluid input/output with adjustment were needed to avoid rapid correction of hyponatremia.
Results: Following tumor diagnosis serum sodium levels dropped from 132 to 118 mEq/l despite fluid restriction up to 30% and administration of furosemide. Hyponatremia was corrected gradually with Tolvaptan administration allowing liberalization of fluid intake and hyperhydration (twice the fluid mantenance rate, chart). The fluid intake was altered based on serum sodium levels and fluid balance. After 5 days of hyperhydration, when the methotrexate was cleared, Tolvaptan doses and frequency was decreased to achieve normal fluid intake and acceptable sodium levels.
Conclusion: Tolvaptan is a safe and effective treatment for severe SIADH when fluid restriction is not possible or effective. However, close monitoring is important to avoid rapid correction of hyponatremia.
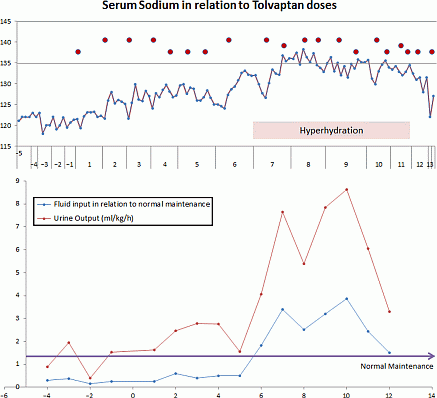
Figure 1




