ESPE2015 Poster Presentations Poster Category 1 Turner & Puberty (11 abstracts)
Improved Determination of Total Serum Estrogenic Bioactivity: Characterisation of Oestrogenic Activity Modulators
Paris Françoise a, , Grimaldi Marina b , Sultan Charles a & Balaguer Patrick b
aDepartement d’Endocrinologie Pédiatrique, Hospital of Montpllier, Montpellier, France; bIRCM, INSERM U896, Montpellier, France
Background: Several years ago, we developed a recombinant cell bioassay to determine serum estrogenic bioactivity (EBA). In addition to its physiological interest, EBA could be a good marker of endocrine-disrupting compounds (EDCs) with estrogenic activity and thus would be useful in the field of environmental-related endocrine diseases.
Aims and objectives: To characterise the type of substances that mediate estrogenic activity.
Methods: We evaluated EBA before and after incubation with the estrogen receptor-α (ER-α) ligand-binding domain. Since ER-α was used in limited amounts, it preferentially captured compounds of high affinity like endogenous estrogens, with a residual EBA being related to low-affinity estrogenic compounds like EDCs. This measure was performed on three sera (S): two from young women during the ovulatory period (S1) and under estroprogestative contraception with 30 μg of ethinyl estradiol (S2), and the third was fetal bovine serum (S3). Radioimmunological determinations of plasma estradiol (E2) were 870, <9 and 25 pg/ml for S1, S2 and S3 respectively.
Results: EBA fell after ER-α incubation for S1 and S2, whereas it remained at 65% of the basal value for S3 (Figure 1).
Conclusions: We confirmed the usefulness of EBA evaluation in physiological conditions to measure both endogenous estrogens and ethinyl estradiol. The evaluation on total serum probably better reflects physiological status than the isolated evaluation of steroids. In addition, the EBA bioassay is able to detect other compounds of lower estrogenic activity and thus may be useful in evaluating environmental-related endocrine diseases.
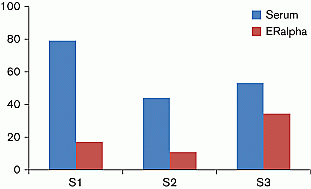
Figure 1 EBA is expressed as the percentage of luciferase activity; the value measured in the presence of 10 nM E2 was taken as 100%.
 }
}



