ESPE2015 Poster Category 2 Gonads (14 abstracts)
Confirmation of Exogenous Serum Estrogenic Activity in a Girl with Premature Thelarche
Paris Françoise a, , Grimaldi Marina b , Sultan Charles a & Balaguer Patrick b
aDépartement d’Endocrinologie Pédiatrique, Hospital of Montpellier, Montpellier, France; bIRCM, INSERM U896, Montpellier, France
Background: The oestrogenic activity of endocrine-disrupting compounds (EDCs) has been reported to be associated with premature thelarche (PT) and precocious puberty. Some years ago, we developed a recombinant cell bioassay to determine serum estrogenic bioactivity (EBA) that is useful in physiology, as well as in the field of the environmental-related endocrine diseases. We recently improved the assay with an evaluation of EBA before and after incubation with estrogen receptor-α (ER-α) ligand-binding domain. Since ER-α is used in limited amounts, it preferentially captures compounds with high affinity, like endogenous oestrogens, with residual EBA being related low-affinity estrogenic compounds like EDCs.
Aims and objectives: To better characterise the high EBA in a 5-year-old girl with PT. This girl had a low radioimmunological plasma estradiol level (<9 pg/ml) contrasting with high EBA (S1) (Figure 1). Her EBA was 86% of the reference value measured in the presence of 10nM E2, which was taken as 100%, i.e., at a pubertal level.
Methods: We reevaluated EBA after incubation of the serum sample with ER-α ligand-binding domain.
Results: EBA remained at 64% of the basal value (S1 ER-α), suggesting that the remaining EBA was potentially due to xenoestrogens (Figure 1). Questioning of the patient’s family revealed that hydrating cream was being applied to her skin for eczema lesions. This cream contained parabens. We hypothesized that the cream might be increasing the EBA, and the basal EBA indeed fell to prepubertal levels, i.e., below 20% (S2) (Figure 1), when her mother switched to a cream without parabens or staining, confirming our hypothesis.
Conclusions: We confirmed the usefulness of our new EBA assay for evaluating environmental endocrine diseases. This should be studied in a higher number of girls with PT.
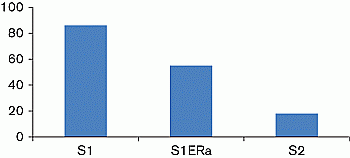
Figure 1 EBA is expressed as the percentage of luciferase activity; the value measured in the presence of 10 nM E2 was taken as 100%.
 }
}



