ESPE2016 Free Communications Syndromes: Mechanisms and Management (6 abstracts)
Whole Exome Sequencing Identifies EPHB4 and PIk3R6 as Causes of Generalized Lymphatic Anomaly
Dong Li a , Tara Wenger b , Christoph Seiler c , Michael March a , Lifeng Tian a , Charlly Kao a , Rahul Pandey a , Kenny Nguyen a , Rosetta Chiavacci a , Patrick Sleiman a, , Maxim Itkin e , Yoav Dori e & Hakon Hakonarson a,
aCenter for Applied Genomics, the Children’s Hospital of Philadelphia, Philadelphia, PA, USA; bDivision of Craniofacial Medicine, Seattle Children’s Hospital, Seattle, WA, USA; cZebrafish Core, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA; dDepartment of Pediatrics, University of Pennsylvania School of Medicine, Philadelphia, PA, USA; eCenter for Lymphatic Imaging and Interventions, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA; fDivisions of Genetics and Pulmonary Medicine, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA
Background: Generalized lymphatic anomaly (GLA) is a rare congenital disorder characterized by aggressive proliferation of dilated lymphatic vessels. The etiology of GLA is poorly understood.
Objective and hypotheses: To identify the underlying genetic basis for GLA.
Method: Exome sequencing (ES) was performed in two families, including a multigenerational family (family-1) with six affected members. RNA-seq was performed on skin biopsies obtained from the lead proband in family-1. To understand the roles of the mutated genes, EPHB4 and PIK3R6 in vascular morphogenesis, we generated two zebrafish models of EPHB4 and PIK3R6 deficiency, respectively.
Results: ES analysis followed by Sanger sequencing in family-1 revealed that 6 affected individuals were heterozygous for an EPHB4 mutation (c.2334+1G>C), a gene previously implicated in a biological pathway related to venous and lymphatic cell fate determination. The mutation was absent in seven unaffected family members. RNA-seq demonstrated that the EPHB4 splice-altering mutation creates a cryptic splice donor that causes the retention of the intervening 12 bp of the intron. For family-2, ES revealed a homozygous variant, c.1393-7C>T, in PIK3R6 in the proband with both parents being heterozygous. PIK3R6 is a regulatory subunit of PI3K complex. Zebrafish knockdown of either EPHB4 or PIK3R6 resulted in vessel misbranching and deformities in the lymphatic vessel development (Figure 1), indicative of possibly differentiation defects both in blood and lymphatic vessels and mimicking the presentations of the GLA patients. Western blot analysis using zebrafish lysates, which contained vascular abnormality, confirmed that reduced EPHB4 signalling resulted in downstream mTORC1 overactivation. Strikingly, drugs that inhibit mTOR signalling were able to rescue this misbranching phenotype.
Conclusion: We report two novel genes that converge on the same physiological PI3K/AKT/mTOR pathway that underlies GLA, presenting a potential avenue for therapeutic intervention for GLA patients.
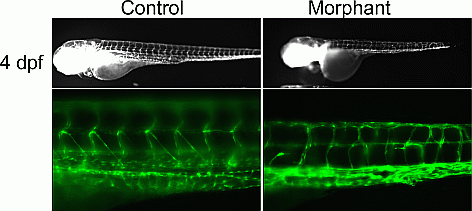
Figure 1 Zebrafish knockdown results in lymphatic developmental defects.
 }
}



